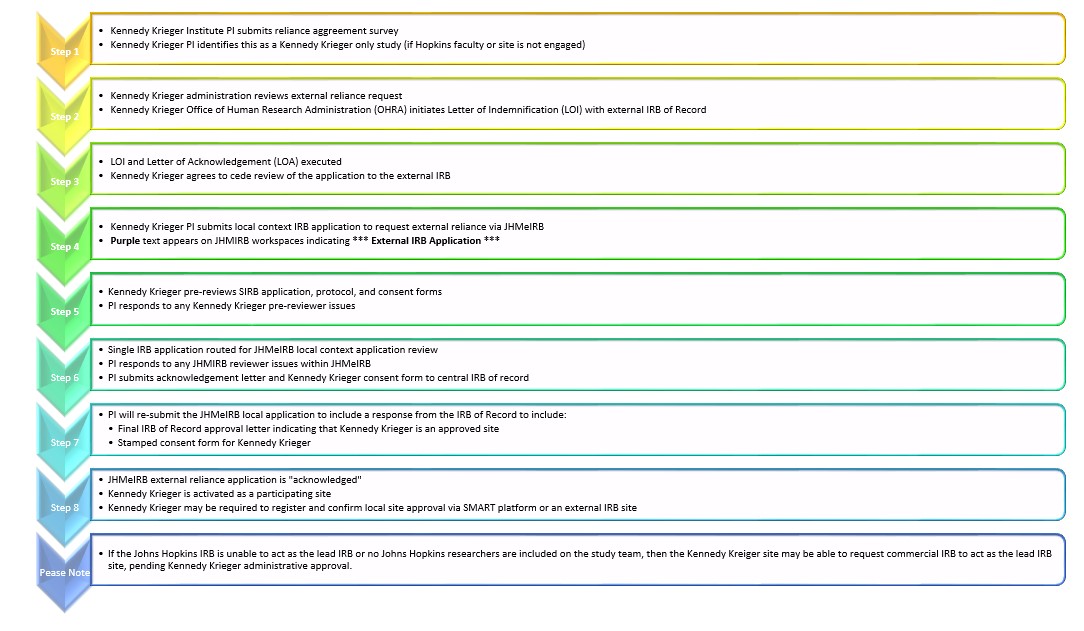
All Kennedy Krieger faculty and staff must submit for JHM IRB review any human subject research or quality improvement project application. In some multi-site non-exempt human research studies, Kennedy Krieger administration may approve reliance on an external IRB other than Johns Hopkins as the single IRB of record. However, a JHM IRB local context application submission with review and approval is still required for these single IRB studies with external reliance. For more information on single IRB, please contact OHRA@kennedykrieger.org.
Single IRB: A Collaborative Process