Research Ethics
The Office of Human Research Administration (OHRA) Research Ethics committee is formed through collaboration with the Kennedy Krieger Institute’s Ethics Committee. This ad hoc committee is composed of Ethics Program members who have broad knowledge of ethical issues and extensive education, training, and experience in helping patients, families, and staff grapple with difficult decisions.
Research studies that pose more than minimal risk have greater potential to negatively affect the safety and rights of research participants, the majority of whom are representative of vulnerable or fragile patient populations. For example, research studies involving gene therapy or first-in-human trials within these populations may warrant additional ethical consideration. In addition, potential ethical dilemmas in research may arise in the context of recruitment, informed consent, child’s assent or dissent, and in the selection and administration of study procedures (proposed or approved studies).
Research protocols that raise particularly complex ethical issues may be submitted to the Research Ethics committee for an ethics consultation at any point in the research process. When such ethical issues arise, the Director of OHRA/Chief Science Officer or a designated OHRA staff member will request a consultation from the Research Ethics committee, which will meet on an ad hoc basis.
The Chairperson of the Ethics Program will reach out to the full membership of the Ethics Program to identify at least three Program member(s), including one community member, who together with an OHRA member will perform the consultation. In addition to the Ethics Program and OHRA members, the Principal Investigator or well qualified designee may be asked to participate. The designated consultant(s) will provide an ethical analysis of the issue and provide ethically permissible options or recommendations to OHRA.
Research Integrity
The Research Integrity committee is responsible for assessing concerns and interpreting policies regarding research integrity and the responsible conduct of research. This committee also ensures that the Institute’s policies and procedures comply with the regulatory requirements of federal funding agencies.
The Chief Medical Officer serves as the Research Integrity Officer and has primary responsibility for implementing the policy if the Institute is presented with allegations of research misconduct. The President of Kennedy Krieger serves as the Deciding Official and consults with the Research Integrity Officer or other appropriate officials to determine whether, among other things, research misconduct occurred.
Applicable regulations include: Public Health Service Policies on Research Misconduct (42 CFR Parts 50 and 93)
High-Risk Research
The High-Risk Research Committee reviews human subjects research studies posing greater than minimal risks to health and/or well-being. Studies that may warrant high-risk review include (1) more than minimal risk clinical trials (e.g., first time listed as PI on a more than minimal risk application; Kennedy Krieger investigator-initiated protocols that are more than minimal risk); (2) challenge protocols (procedures in this protocol that attempt to induce new symptoms in research participants); (3) biopharmaceutical company concerns (e.g., new biopharmaceutical; biopharma with a single drug product; recent regulatory concerns; (4) special study populations; and (5) vulnerable populations. The Committee meets on an ad hoc basis.
Outside Interests
The Outside Interests committee reviews disclosures of outside interests and makes determinations regarding the resolution of actual or perceived conflicts of interest related to research, consistent with applicable federal regulations, institutional policies and procedures, and the policies of the Johns Hopkins University School of Medicine. This committee aims to maintain objectivity in the design, conduct, and reporting of research; foster transparency; and best serve the rights and welfare of participants enrolled in research.
Applicable regulations include the US Department of Health and Human Services Public Health Service regulations on Responsibility of Applicants for Promoting Objectivity in Research for which PHS Funding is Sought (42 C.F.R. Part 50, Subpart F), which is commonly known as the financial conflict of interest (FCOI) regulation.
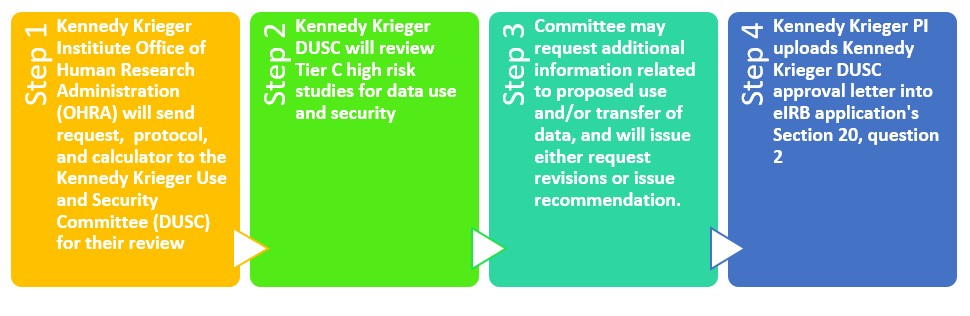
Data Use & Security
As part of its pre-review process of human subjects research studies, OHRA may request recommendations from the Data Use and Security Committee (DUSC) on issues related to access, use, and disclosure of protected health information (PHI) for human subjects research purposes. Any data outputs sought with the intent of conducting human research must be vetted through the OHRA and an appropriate IRB number must be granted prior to submitting data output requests from the Institute’s Enterprise Intelligence team.
Studies that may warrant DUSC review include, but are not limited to, protocols proposing to share 500 or more records with a third party or the transfer of data outside of the Institute; utilize a live data feed from an enterprise clinical system; collect Protected Health Information (PHI) collection via an app; or create a research registry.
Risk Tiers Matrix Calculator
The Kennedy Krieger Institute PI includes the application number and the name of the project PI. An algorithm calculates the study’s risk, based on the answers to eight questions including data storage, PHI/sensitive data, data analysis, number of data records, data transfer, consenting, apps, websites, and electronic feeds. After the questions have been answered, a risk tier appears on the document (A = Low risk, B = Minimal risk, or C = High risk).
Human Biospecimen Transfer Review
Kennedy Krieger Institute’s Biospecimen Transfer Review Committee reviews researchers’ requests to transfer human biospecimens obtained for clinical and/or research purposes to collaborators outside of the Institute. The Office of Human Research Administration (OHRA) coordinates this review process.
OHRA is also available to assist researchers in preparing and executing a Material Transfer Agreement (MTA) and any other documentation that may be required to facilitate the transfer in accordance with institutional and IRB requirements. For more information, please contact OHRA@kennedykrieger.org.
Kirby Center Protocol Review
The Kirby Center Protocol Review Committee (PRC) reviews all proposals that utilize the resources of the F.M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute. The members of the PRC have experience and expertise in all areas of functional imaging, including paradigm design, MR imaging, spectroscopy, and EEG methodology.
