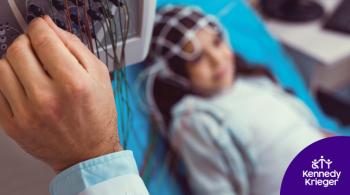
Two researchers at Kennedy Krieger Institute received $5.5 million to start an innovative three-year neurorehabilitation program aimed at improving the brain health of pediatric cancer patients with brain tumors.Learn More

Kennedy Krieger’s scientists and researchers have made crucial discoveries, leading to innovative treatments, enhanced diagnosis, patient care and student education for individuals with disorders of the nervous system. This work is possible because of our research participants, who play a pivotal role in helping make new discoveries.
Participating in research is a way to help researchers learn more about certain health and learning problems. When you enroll yourself or your child in a clinical trial or research study, you become a partner in scientific discovery. These critical discoveries, such as new treatments and breakthroughs, impact the patients and students we serve.
Ultimately, your contribution can help future generations lead healthier lives. Major breakthroughs could not happen without the generosity of research participants.
Kennedy Krieger seeks research participants of all ages. While specific criteria for diagnosis or age varies by study or trial, research at Kennedy Krieger is collectively open to all ages and abilities. This includes adults, typically developing children and adolescents, and children and adolescents with disorders of the nervous system.
Get started on your journey to pivotal scientific discovery by enrolling in a research study or clinical trial.
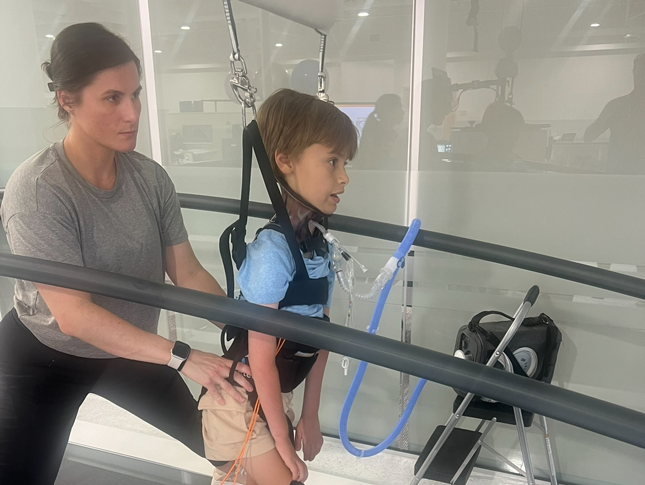

Two researchers at Kennedy Krieger Institute received $5.5 million to start an innovative three-year neurorehabilitation program aimed at improving the brain health of pediatric cancer patients with brain tumors.Learn More

Kennedy Krieger Institute has been awarded an $8.17 million grant from the National Institutes of Health to establish and lead a first-of-its-kind network in the U.S. dedicated to advancing treatments for Batten diseases, a group of rare pediatric neurodegenerative disorders.Learn More

Kennedy Krieger Institute has been awarded a highly competitive five-year, $6.25 million P50 Center Grant from the National Institutes of Health (NIH) and Eunice Kennedy Shriver National Institute of Child Health and Human Development to create the Precision Rehabilitation Across the Lifespan Center.Learn More
The biggest benefit of participating in research is being a partner in scientific study. When you or your child enroll in a study or trial, you help experts at Kennedy Krieger discover treatments and breakthroughs that benefit the patients and students we serve. This, in turn, yields significant progress that allows future generations to live healthier lives.
While some studies or trials may offer compensation in the form of money or gift cards, Kennedy Krieger does not guarantee that these benefits are available in all studies or trials. If you have questions about compensation for a specific study or trial, please refer to the contact information associated with that project.
Kennedy Krieger goes to great lengths to ensure the safety and care of research study and trial participants. Each individual participating in a clinical trial or research study at Kennedy Krieger receives expert assessment and is carefully monitored throughout the study. All participants, or their parents or guardians, are given specific details—including any known risks or potential benefits—before agreeing to participate in the study or trial. This process is known as informed consent.
All research is undertaken with the utmost care and concern for the safety, health, confidentiality and privacy of each participant. Every study and clinical trial is approved and monitored by the Johns Hopkins Institutional Review Board—an independent committee that ensures the trial is ethical and that the rights of participants are protected—and many are also monitored by the Food & Drug Administration. We monitor each participant closely via multiple on-site clinic visits. In addition, each participant is provided multiple contact numbers to report concerns in between visits.
Kennedy Krieger encourages typically developing children and adolescents to participate in research. While some of our studies or trials seek only children and/or adults with a certain diagnosis, numerous studies and trials are actively recruiting typically developing children and adolescents. You can learn more about them here.
Clinical research is the study of health and illness in people. It is the way we learn how to prevent, diagnose and treat illness. Clinical research goal is to move findings from the bench (done in labs) to the bedside into developing new treatments and information that will benefit patients. Clinical trials, research in epidemiology, physiology and pathophysiology, health services, education, outcomes and mental health can all fall under the clinical research umbrella.
A clinical trial is a type of clinical research study. A clinical trial is an experiment designed to answer specific questions about possible new treatments or new ways of using existing (known) treatments. Clinical trials are done to determine whether new drugs or treatments are safe and effective. Clinical trials are part of a long, careful process which may take many years to complete. First, doctors study a new treatment in the lab. Then they often study the treatment in animals. If a new treatment shows promise, doctors then test the treatment in people via a clinical trial.
People often confuse clinical research or clinical trials with medical care. This can be especially confusing if your doctor is also the researcher. When you receive medical care from your own doctor, he or she develops a plan of care just for you. When you take part in a clinical research study, you and the researcher must follow a set plan called the “study protocol.” The researcher usually can’t adjust the plan for you – but the plan includes steps to follow if you aren’t doing well. It’s important to understand that a clinical trial is an experiment. By its nature, that means the answer to the research question is still unknown. You might or might not benefit directly by participating in a clinical research study. It is important to talk about this topic with your doctor/the researcher.
For information on clinical trials, please call our Clinical Trials Unit at 443-923-3850. If you have questions about a specific study or trial, please refer to the information provided on the study or trial’s flyer or webpage.

Get started on your journey to pivotal scientific discovery by enrolling in a research study or clinical trial.