Modern machine learning has proven highly effective for unsupervised problems. We are developing algorithms that combine data-driven and physics-based models to improve the estimation of underlying MRI structures. These methods enhance accuracy, reduce the number of required measurements, and provide a robust framework for extracting meaningful information and biomarkers from latent variables. A key limitation in quantitative MRI is the trade-off between resolution, SNR, and scan time. Long acquisitions may improve image quality but are impractical in clinical settings due to patient discomfort and motion artifacts. As multi-contrast imaging becomes more common, reducing scan time is increasingly important. To address this, we develop methods that minimize measurement needs while preserving accuracy. Instead of traditional, compute-heavy optimization, we use data-driven models (e.g., deep CNNs) that approximate solutions with parametric functions. Though training is resource-intensive, it is done offline, allowing fast and efficient deployment in practice.
We developed deep learning frameworks to solve ill-posed inverse problems in neuroimaging. Recently focusing on quantitative susceptibility mapping (QSM) and susceptibility tensor imaging (STI), we introduced Learned Proximal Networks (LPNs)1 and a novel proximal matching training strategy, enabling accurate, interpretable reconstructions from limited data. We introduced DeepSTI2, which reconstructs full susceptibility tensors using fewer orientations, and WaveSep3, a tool for separating diamagnetic and paramagnetic sources to improve specificity in neurodegeneration studies. We also developed ProxiMO4, an unsupervised approach that trains deep networks on single-orientation QSM data without requiring ground truth, expanding applicability and efficiency.
Building on our work with Learned Proximal Networks (LPNs), we are creating deep learning tools that not only reconstruct high-quality images but also quantify uncertainty and reveal the patterns learned from data. We are developing advanced computer models to detect and characterize differences between healthy and diseased brain structures using MRI. We will extend these methods using diffusion models to better handle noisy inputs and train on both healthy and disease-specific scans to identify data-driven biomarkers. These models aim to improve image reconstruction, interpretability, and diagnostic precision in MRI.
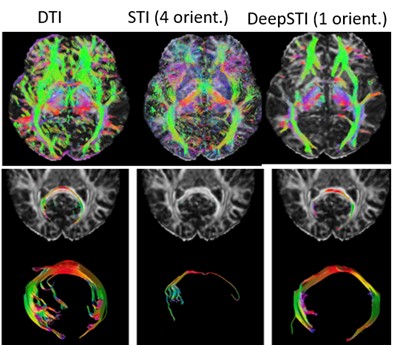
Color images coding directions of white matter tracts and tractography with DTI, STI with 4 head orientations, and with our DeepSTI2
- Fang, Z., Buchanan, S. & Sulam, J. What’s in a prior? learned proximal networks for inverse problems. Int. Conf. on Learn. Represent. (2024).
- Fang, Z., Lai, K.-W., van Zijl, P., Li, X. & Sulam, J. Deepsti: towards tensor reconstruction using fewer orientations in susceptibility tensor imaging. Med. image analysis 87, 102829 (2023).
- Fang, Z., Shin, H.-G., van Zijl, P., Li, X. & Sulam, J. Wavesep: A flexible wavelet-based approach for source separation in susceptibility imaging. In: International Workshop on Machine Learning in Clinical Neuroimaging, 56–66 (Springer, 2023).
- Orenstein, S. et al. Proximo: Proximal multi-operator networks for quantitative susceptibility mapping. In International Workshop on Machine Learning in Clinical Neuroimaging, 13–23 (Springer, 2025).
