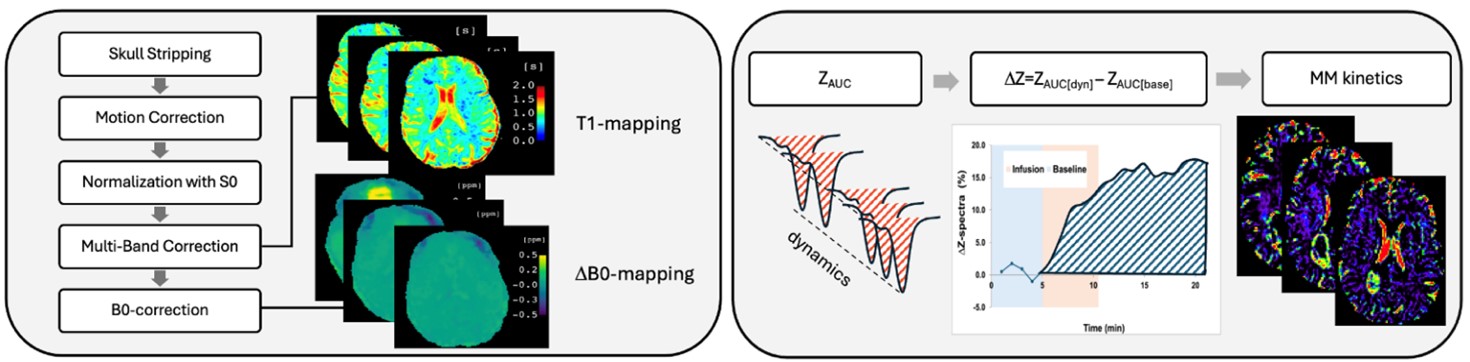
Intravenous (i.v.) Gadolinium based contrast agent (GBCA) enhanced MRI to measure CSF circulation and clearance
To date, GBCA-enhanced MRI has been used mainly for studying blood circulation and perfusion. Our goal is to establish i.v. GBCA MRI for CSF studies in a way analogous to intrathecal GBCA MRI. We previously developed an MRI approach for dynamic imaging of GBCA distribution in the CSF in human brains, termed dynamic susceptibility contrast in the CSF (cDSC) MRI. Using cDSC MRI, we demonstrated that dynamic CSF distribution can be measured in various brain regions in healthy subjects.
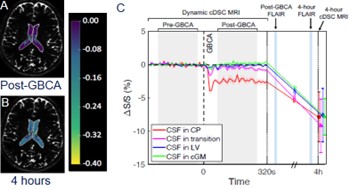
Choroid plexus (CP) and ventricle: The CP is a key component in the CSF system and often considered the starting point of CSF circulation in the brain. Our study showed that the GBCA entered CSF via the CP ~20 s after i.v. injection, gradually spreading across the LV over time.
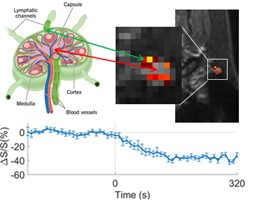
Lymph nodes are considered the final destination for CSF clearance from the brain. Our study showed that significant cDSC signal changes were observed post-GBCA at the surface area of lymph nodes, where lymphatic channels are located.
We will continue to develop the method with the goal of establishing intravenous GBCA MRI for studying CSF circulation in human brains in a similar way to intrathecal GBCA MRI. We will perform longitudinal cDSC MRI scans at multiple time points after i.v. administration to establish: 1) the entry points and pathways for i.v. GBCAs to enter the CSF circulation; and 2) the distribution and clearance of GBCAs in the CSF space in various brain regions over time. The resulting data will provide an important baseline for studies using i.v. GBCA to investigate CSF dysfunction in various brain diseases. Note that only one single standard dose of i.v. GBCA is needed in these studies.
Dynamic glucose enhanced (DGE) imaging
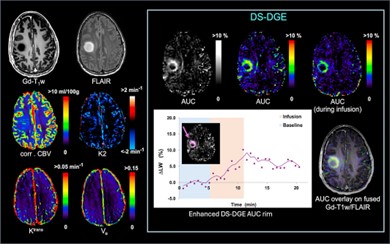
We recently developed a novel DGE MRI approach that exploits the T2-relaxation features of the direct saturation (DS) water spectrum. DS-DGE MRI can assess the uptake and transport of D-glucose and is inherently independent of B0 shifts occurring between the dynamics. We will further develop the DGE method so that our Z-spectra data now includes the asymmetric broadening together with the T2 effects. This will allow us to detect hydroxyl protons in the intermediate to fast exchange condition, thereby increasing the effect size. The figure shows a first example of the use of this method in brain tumors.
We will develop a new 3-tissue-compartment model using Michaelis-Menten (MM) kinetics to quantify physiologically relevant parameters for BBB transport and metabolism. The data processing will be improved by designing a pipeline that includes multiple steps including the MM kinetics. This will improve the applicability of DGE MRI and provide a more accurate and physiologically meaningful tissue characterization.