Magnetic susceptibility (x) is a parameter that describes how much a material becomes magnetized in response to an external magnetic field. In brain tissue, x reflects the combined contribution from paramagnetic iron and diamagnetic myelin. Brain iron sources include iron bound to hemoglobin in blood (heme-iron) and non-heme iron stored mostly in cellular ferritin, e.g. in neurons and microglia. The magnetic susceptibility of heme-iron depends strongly on its oxygenation status, with deoxy-hemoglobin being paramagnetic. In comparison, myelin is the most abundant diamagnetic source in brain white matter. Accurate and specific measures of local brain iron and myelin content, and oxygenation status have the potential to serve as important biomarkers of brain pathophysiological changes during development, aging, and neurodegeneration.
Quantitative susceptibility mapping (QSM) enables in vivo mapping of tissue magnetic susceptibility,1 and has been used widely to assess brain iron content changes in various neurodegenerative disorders. However, QSM only measures bulk tissue magnetic susceptibility, but cannot differentiate spatially colocalized iron (paramagnetic) and myelin (diamagnetic) content within an MRI voxel or differentiate heme-iron versus non-heme iron contributions except in large vessels.2
Recent developments have enabled separate estimations of paramagnetic and diamagnetic tissue sources by combining QSM and relaxometric measures (R2’ or R2*), or by modeling the complex gradient echo (GRE) signal evolution over time3,4. Such QSM source separation has recently led to an approach for predicting remyelination capacity based on tissue iron content in acute multiple sclerosis (MS) lesions (see figure).5
We are developing a next-generation susceptibility source separation framework to isolate contributions from heme-iron, non-heme iron, and myelin—enabling more specific biomarkers of brain physiology and pathology.
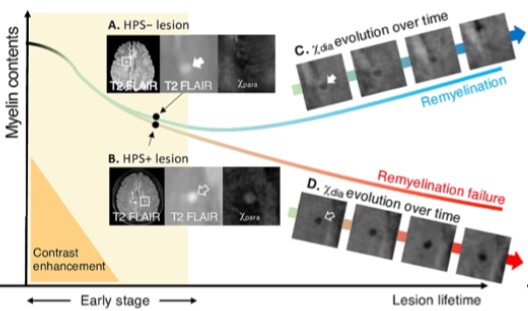
Figure: High-paramagnetic signs (HPS) observed on the paramagnetic susceptibility (Xpara) in early-stage multiple sclerosis (MS) lesions may help differentiate their subsequent remyelination outcomes. Demyelinated lesions with normal or less iron deposition (HPS- lesion) typically demonstrate successful recovery of myelin contents (remyelination), indicated by an increase in diamagnetic myelin signal (Xdia). From Shin H. et al, Neuroimage Clin. 2025;24:103748.
Reference:
- Committee QSMCO, Bilgic B, Costagli M, Chan KS, Duyn J, Langkammer C, Lee J, Li X, Liu C, Marques JP, Milovic C, Robinson SD, Schweser F, Shmueli K, Spincemaille P, Straub S, van Zijl P, Wang Y, Group IE-MTPS. Recommended implementation of quantitative susceptibility mapping for clinical research in the brain: A consensus of the ISMRM electro-magnetic tissue properties study group. Magn Reson Med. 2024;91(5):1834-62.
- Wehrli FW, Fan AP, Rodgers ZB, Englund EK, Langham MC. Susceptibility-based time-resolved whole-organ and regional tissue oximetry. NMR Biomed. 2017;30(4).
- Shin HG, Lee J, Yun YH, Yoo SH, Jang J, Oh SH, Nam Y, Jung S, Kim S, Fukunaga M, Kim W, Choi HJ, Lee J. chi-separation: Magnetic susceptibility source separation toward iron and myelin mapping in the brain. Neuroimage. 2021;240:118371.
- Chen J, Gong NJ, Chaim KT, Otaduy MCG, Liu C. Decompose quantitative susceptibility mapping (QSM) to sub-voxel diamagnetic and paramagnetic components based on gradient-echo MRI data. Neuroimage. 2021;242:118477.
- Shin HG, Kim W, Lee JH, Lee HS, Nam Y, Kim J, Li X, Zijl PCMV, Calabresi PA, Lee JH, Jang J. Association of iron deposition in MS lesion with remyelination capacity using susceptibility source separation MRI. Neuroimage-Clin. 2025;45.
