Chemical Exchange Saturation Transfer (CEST) MRI has emerged as a powerful technique for detecting low-concentration metabolites and proteins by indirectly enhancing their signals through the water signal used in conventional MRI. CEST exploits the exchangeable protons in metabolites—such as guanidinium protons in creatine (Cr, 2.0 ppm from the water signal) and phosphocreatine (PCr, 2.5 ppm), and amide protons in mobile proteins (3.5 ppm). These protons exchange with bulk water, generating a detectable contrast. A typical CEST experiment requires acquiring a full water saturation spectrum (Z-spectrum) by collecting images at multiple saturation frequency offsets. However, this process is time-consuming and prone to motion artifacts. Furthermore, isolating specific CEST contrasts from the myriad of exchanging protons in tissue presents a significant challenge. To address these limitations, we developed a suite of methods targeting both rapid acquisition and improved signal extraction. First, we implemented a motion-robust readout using 3D stack-of-spirals (SOS) gradient echo (GRE), which offers high sensitivity and full-brain coverage in a clinically feasible scan time (~5–7 minutes). This approach combines the speed of spiral imaging with the volumetric efficiency of 3D acquisition, delivering high spatial and temporal resolution while improving robustness to motion and scan-rescan reproducibility. For signal quantification, we introduced the Polynomial and Lorentzian Line-shape Fitting (PLOF) method, which effectively suppresses confounding contributions from direct water saturation and magnetization transfer. PLOF enables specific extraction of Cr, PCr, and amide CEST signals in both brain and muscle.
We applied this PCr/Cr CEST approach to human skeletal muscle using an in-magnet plantar flexion exercise paradigm. This model leverages the well-established metabolic conversion of PCr to Cr during muscle activity, enabling real-time visualization of energy metabolism using CEST MRI. Together, these innovations significantly advance the reliability, speed, and clinical utility of CEST imaging.
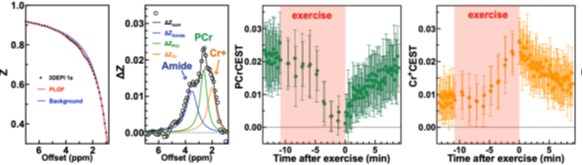
Representative Z-spectrum (box 1) and CEST (Z-difference) signals (box 2) from the exercise-activated gastrocnemius muscle, acquired using a rapid CEST protocol during and after in-magnet plantar flexion exercise. PCr levels showed a marked decrease during exercise with gradual recovery post-exercise (box 3), while Cr⁺ levels increased during exercise and declined during recovery (box 4).
Ziqin Zhang+, Kexin Wang+, Sooyeon Park, et. al.“ The exchange rate of creatine CEST in mouse brain”, Magnetic Resonance in Medicine, (2023) 90 (2), 373-384.
Kexin Wang, Jianpan Huang, Licheng Ju, et. al. “ Creatine mapping of the brain at 3T by CEST MRI”, Magnetic Resonance in Medicine, (2024) 91 (1), 51-60.
Licheng Ju+, Kexin Wang+, Michael Schär, et. al. “ Simultaneous creatine and phosphocreatine mapping of skeletal muscle by CEST MRI at 3T”, Magnetic Resonance in Medicine, (2024) 91 (3), 942-954.
Kexin Wang, Licheng Ju, Yulu Song, et. al. “ Whole-Cerebrum guanidino and amide CEST mapping at 3T by a 3D stack-of-spiral gradient echo acquisition.”, Magnetic Resonance in Medicine, (2024) 92 , 1456.
Licheng Ju, Michael Schär, Kexin Wang, et. al. “ Mitochondrial Oxidative Phosphorylation Capacity in Skeletal Muscle Measured by Ultrafast Z-Spectroscopy (UFZ) MRI at 3T”, Magnetic Resonance in Medicine, (2025) 93, 1273-1284.
